Photoelectric effect#
What you need to know
Photoelectric effect: Electrons are ejected when light is shone onto a material. This experiment puzzled 20th century scientists who thought of light as being a continuous stream of waves.
Only by abandoning classical thinking and accepting the reality of quantized energy reconciles experiments with theory.
Photoelectric effect contradicts classical mechanics.#
To reconcile experiment with theory Planck had to assume that a heated material absorbs and emits light with discrete energies: \(0, h\nu, 2h\nu, 3h\nu, …\). At a time this discreteness was thought to be nothing more than a temporary mathematical trick to fix theory.
Einstein, on the other hand, was more imaginative and saw in Plank’s prescription more than just a math trick. He suggested that light can behave like a stream of particles with discrete countable energy packets which he called photons. This view was instrumental in making sense of the photoelectric experiment.
Thus experiments showed that energies of both matter and light are quantized!
Photoelectric effect#
When you shine a light with sufficient energy content (UV radiation) on a metal surface electrons, start flying off the surface. This is the essence of the photoelectric effect. Observation of the photoelectric effect was crucial for showing that only quantum mechanics can make sense of how light interacts with the matter.
Applications of photoelectric effect#
Besides its historical role in the establishment of QM photoelectric effect has many practical applications. It is relevant for the design of solar cells, photovoltaics, photoelectron spectroscopy, night vision, etc.

Classical mechanics fails to explain photoelectric effect#
Early experiments of photoelectric effect appeared truly puzzling to scientists of the 20th century for the following reasons:
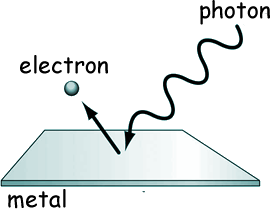
They expected to see more electrons being ejected when increasing the intensity or duration of radiation. According to CM if one shines the light long enough or increases the intensity by pumping more energy then surely those pesky electrons will start flying. (reminder: Intensity of light is a quantity measuring the amount of energy transferred per unit surface per unit time.)
That was not at all what experiments showed. Unless radiation had a frequency above a certain threshold \(\nu_0\) not a single electron would escape regardless of the intensity of radiation. That was really weird.
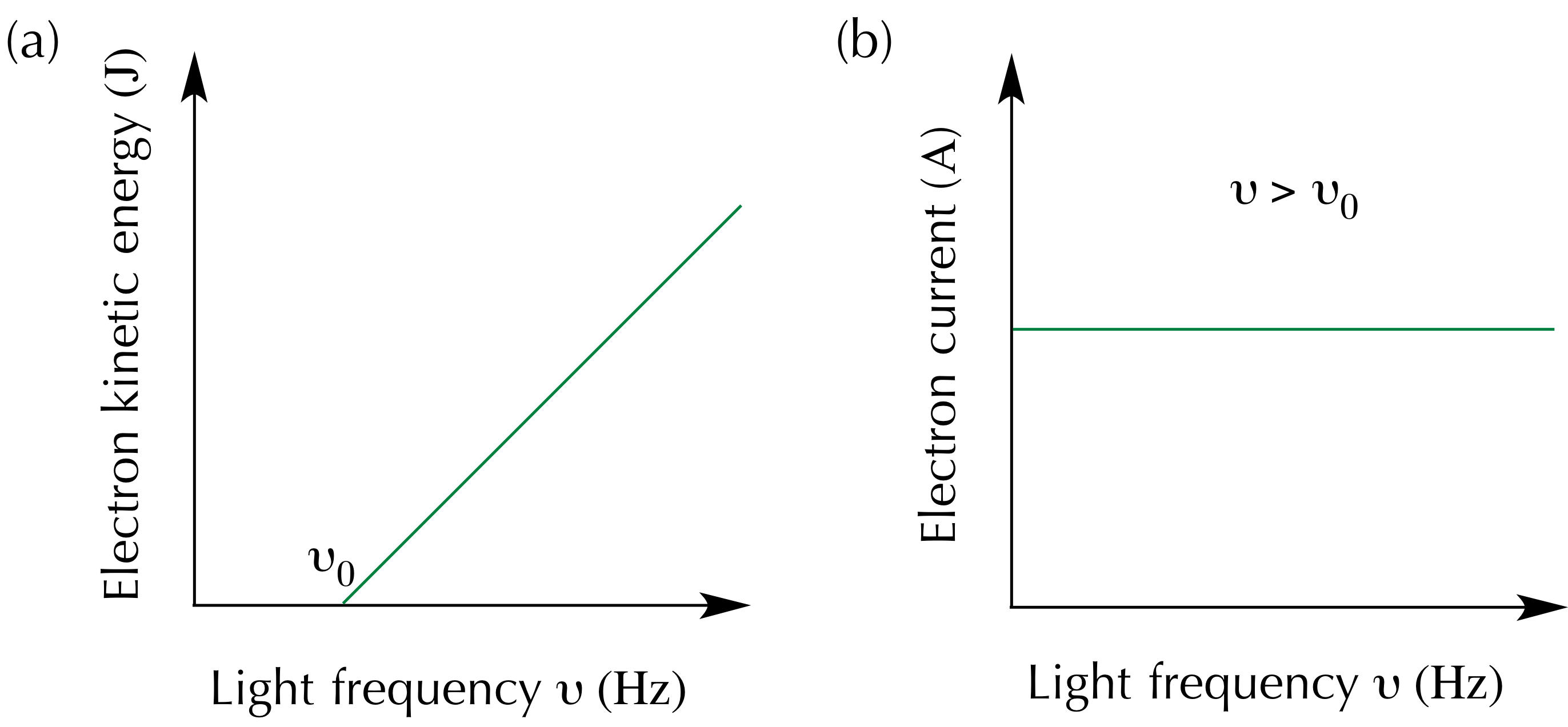
In striking contradicton with classical mechanics electrons get ejected immediately once the frequency threshold is crossed even if the intensity of the radiation is minimal.
Furthermore, the kinetic energy of an ejected electron is a linearly increasing function of the frequency of light with no dependence on the intensity.
Photons explains photoelectric effect#
Light consists of photons: tiny packets of energy carrying \(h\nu\) energy.
Intensity of light is measure of number of photons. frequency is measure of energy of photons.
1 photon can collide with 1 electron and eject it if it has sufficient energy
Any extra energy gets converted into kinetic energy of ejected electron
If frequency is lower than threshold photon does not transfer any energy to electron!